In vitro services in
neurodegenerative diseases (NDD)
Using our unique know-how in the solubilization and stabilization of oligomeric proteins (AβO, αSO and TauO), ETAP-Lab is offering a new medium-throughput in vitro screening pharmacology service in the field of evaluating proteopathic neurodegenerative diseases.

Unique proprietary know-how
ETAP-Lab has a unique know-how to produce and stabilize oligomer aggregates from their human monomers. Oligomer preparations are perfectly characterized, both at the physico-chemical level and in terms of their neurotoxicity.
Trusted in vitro models
All the models proposed are highly reproducible and validated with reference molecules. ETAP-Lab guarantees the validity of each test, using quantifiable internal controls. We are also certified to ISO 9001:v2015.

Expertise & scientific relevance
Our models are based on recent scientific data, which demonstrates the involvement of oligomers in the early stages of neurodegenerative diseases. Thanks to our team of neurobiologists and pharmacologists, we are able to offer a high level of scientific support.
They trust ETAP-Lab
Thank you very much for your professional services in in vitro neurodegenerative studies. We have been collaborating with ETAP-Lab for the past years. You have delivered the services in a professional manner within the approved timelines and with a high quality. Furthermore you have been very proactive to resolve technical related queries. It has been such a great pleasure working with you and we are looking forward to build a long term relationship with you!
Dr. Yuhong Dong, CSO & Co-founder, SunRegen Healthcare AG
Oligomer-based in vitro models
of NDD A relevant paradigm for your drug screening
Our drug screening service now enables you to test and select your innovative compounds in models based on the latest advances in the understanding of the mechanisms underlying neurodegenerative diseases.
Indeed, for several years now, evidence has been accumulating on the involvement of the oligomers β-amyloid and tau for Alzheimer’s disease and alpha-synuclein for Parkinson’s disease. Several ongoing clinical studies targeting these oligomers are now showing encouraging results, and could ultimately lead to the first treatments capable of curbing the development of these pathologies.
Our drug screening service is based on our unique know-how in the production and stabilisation of oligomers from human monomers. Each batch of oligomer produced is identified, and its composition and toxicity are evaluated before being used in your studies.
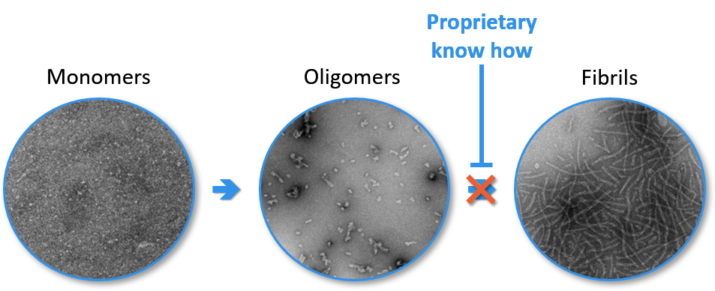
All our tests have been rigorously validated, demonstrating the reproducibility and reliability of the proposed experimental situations.
Example of characterization
Coomassie blue staining of a Aβ 1-42 oligomers preparation in SDS-PAGE
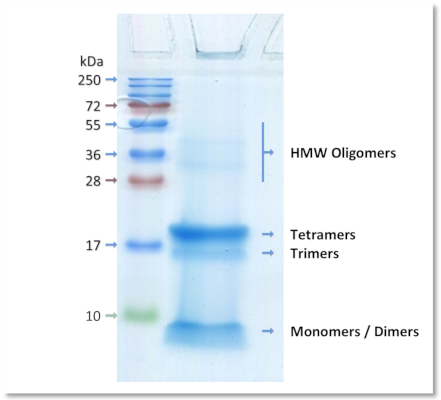
Dot-blot detection of Aβ 1-42 oligomers
Human oligomeric Aβ 1-42 preparations were separated using 15% SDS-PAGE gel under non-reducing conditions. The gel profile revealed by Coomassie blue staining demonstrates that oligomers contain a mixture of stable dimers, trimers and tetramers, as well as traces of high molecular weight oligomers.
Aβ 1-42 oligomers and Aβ 1-42 fibrils were probed with the Rabbit Anti-oligomer (A11) antibody, which recognizes all types of oligomers, but not fibrils, as shown in our preparation.
Our models
Alzheimer's disease
In vitro Amyloid-beta oligomers model
This model, which uses our β-Amyloid 1-42 (AβO) oligomer preparation, allows in vitro detection of the activity of molecules for neuroprotective or immunotherapy purposes, for Alzheimer’s disease. These tests can be carried out in pre-treatment (before the addition of AβO) or in co-incubation on primary cultures of cortical neurons from rats or mice.
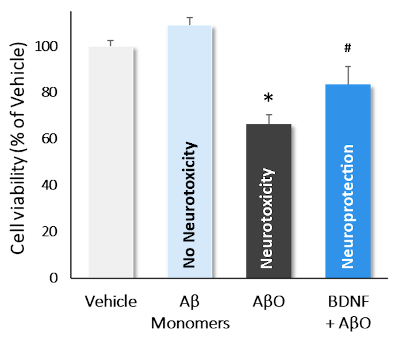
Incubation of cortical neurons with Aβ monomers, AβO and AβO+BDNF . AβO decreased quite significantly cell viability (*; p<0.01*; p<0.01), while monomers showed no neurotoxic effect. Incubation with BDNF significantly reversed AβO-induced neurotoxicity (#; p<0.01). Data are expressed as percent of vehicle (set at 100%) and represent the mean ± SD. (N=3, n=12).
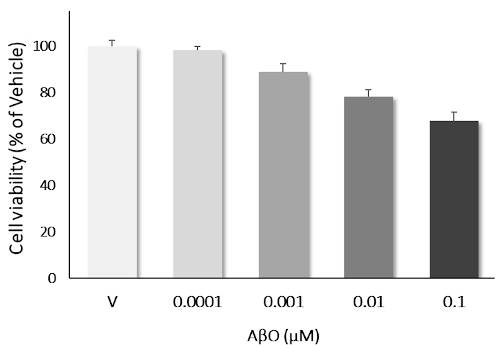
AβO induces dose-dependent neurotoxicity: Primary cortical neurons challenged with AβO over the concentration ranges of 0.1 nM to 0.1 μM, showed a clear dose-dependant neurotoxicity. Data are expressed as percent of vehicle (set at 100%) and represent the mean ± SD. (N=3, n=12).
In vivo Amyloid-beta oligomers model
Based on the toxicity of A-beta oligomers, we have developed a model for Alzheimer’s disease, the effects of which are visible in elderly subjects just as in humans. Explore our model through the poster presented in Algarve in 2023 during the FRM 2023 event organized by the FENS.
Parkinson's disease
In vitro alpha-synuclein oligomers model
This model, using our preparation of alpha-synuclein oligomers (ASO), makes it possible to detect the activity of molecules in vitro for neuroprotective or immunotherapy purposes for Parkinson’s disease. These tests can be carried out in pre-treatment (before the addition of ASO) or in co-incubation on primary cultures of striatal neurons from rats or mice.
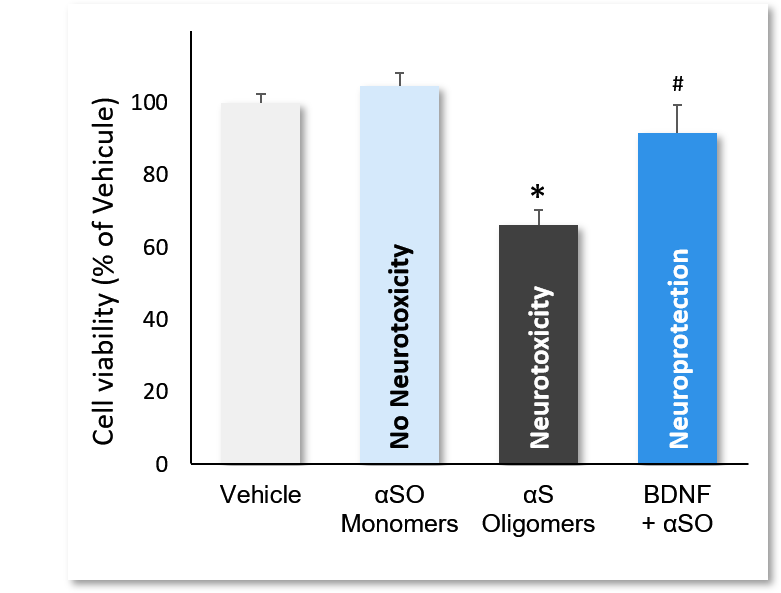
Incubation of cortical neurons with αS monomers, αSO and αSO+BDNF. αSO decreased quite significantly cell viability (*; p<0.01), while monomers showed no neurotoxic effect. Incubation with BDNF significantly reversed ASO-induced neurotoxicity (#; p<0.01). Data are expressed as percent of vehicle (set at 100%) and represent the mean ± SD. (N=3, n=12).
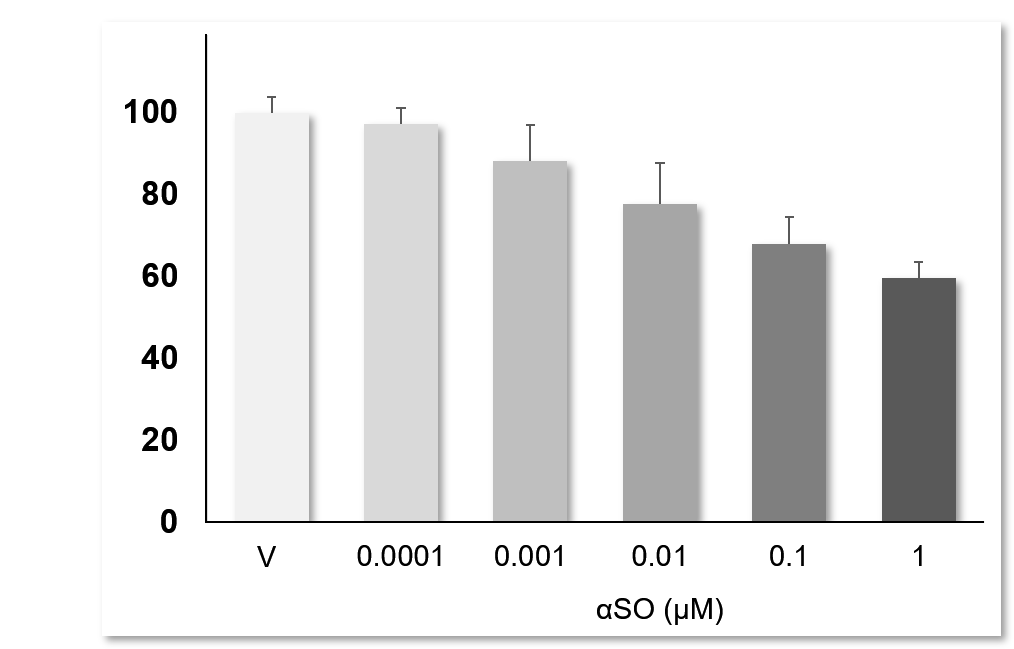
αSO induces dose-dependent neurotoxicity: Primary striatal neurons challenged with αSO over the concentration ranges of 0.1 nM to 0.1 μM, showed a clear dose-dependant neurotoxicity. Data are expressed as percent of vehicle (set at 100%) and represent the mean ± SD. (N=3, n=12).





