Newsletter #8: The ETAP-Lab Psoriasis Model
The psoriasis model induced by the chronic application of imiquimod (IMI) proposed by ETAP-Lab reproducibly replicates the key markers of the pathology observed in humans: hyperproliferation of keratinocytes, hyperkeratosis and parakeratosis. At the systemic level, the strong activation of the immune system alongside the increased expression of characteristic cytokines such as IL-6, Il-17, IL-23 and TNF-alpha is also consistent with human pathology. Psoriasis is not, however, limited to these parameters classically measured in animals. Indeed, the skin discomfort induced by psoriasis (itching and aesthetic aspects) is central to complaints from patients in the clinical setting. For this reason, ETAP-Lab has set up innovative behavioural tests and visual scoring that allow measurement of these parameters, which both increase model predictivity and broaden the scope of the results (well-being, detection of side effects, etc.).
Contents
1.Model Predictivity
2.Relevant assessment tools
a. PASI Score: the objectification of macroscopic observations
b. Histological and biochemical analysis
c. Analysis of animal welfare and behaviour
3. Illustration of a study carried out by ETAP-Lab
1. Model Predictivity
Clinically-proven reference molecules from different pharmacological classes are available to validate our trials, providing a point of comparison for your molecules in development. We were able to test the effects of four reference treatments via three administration routes, as shown in Figure 1. All these treatments significantly reduce the pathology markers induced by the IMI, in comparison with the data obtained in the control groups, as detailed below.
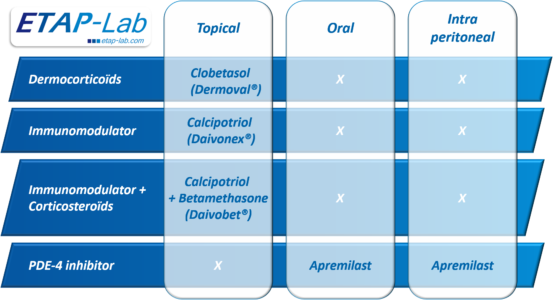
Figure 1: Reference treatments tested on the ETAP-Lab IMI-induced psoriasis model
2. Relevant assessment tools
a. PASI Score: the objectification of macroscopic observations
To evaluate the effects of active molecules in this model, we use exactly the same scoring scale as is used in human clinics: the PASI. The use of the clinical score facilitates the interpretation and understanding of the preclinical results by medical experts and health authorities during the examination of your preclinical file.
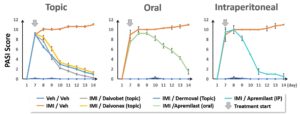
Figure 2: Graphic representation of PASI score averages according to treatment administered and route of administration (From Day 8 to Day 14, for all administration routes, IMI/Veh groups differ significantly from Veh/Veh groups. Reference treatments administered by topical, oral or intraperitoneal route significantly reduce the PASI Score).
The PASI scale takes account of the macroscopic aspect by evaluating the intensity of skin erythema, indurations and desquamation. These three descriptive parameters allow a sensitive evaluation of the effects of active molecules.
As shown in Figure 2, the use of PASI as a preclinical assessment tool provides a measure of changes in treatment efficacy that is both highly reproducible and predictive. This score accurately reflects the skin’s general appearance, as shown in Figure 3.
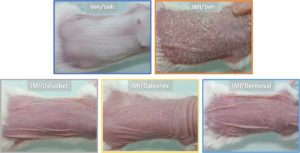
Figure 3: Mice groups by topical treatment, representative photographs taken at the end of the experiment
b. Histological and biochemical analysis
As in all dermatology models, histological analyses provide important evidence for characterisation of the effects of the pathology and these treatments. In the case of psoriasis, these analyses focus on the level of dermal skin inflammation and the hyperproliferation of keratinocytes, hyperkeratosis and parakeratosis.
Working jointly with an anatomopathologist specialized in skin pathology has enabled us to establish a microscopic severity score for psoriasis that is unique. Beyond a simple qualitative analysis, the scoring developed by ETAP-Lab allows quantification of the histological alterations, so that the effects of treatments can be compared, using statistical analyses.
The biological investigations are completed using blood and tissue determinations of the expression of inflammation markers. The reference treatments reduce the expression of blood markers of inflammation, notably Il-17 and IL-23, which are specific markers of psoriasis (Figure 4).
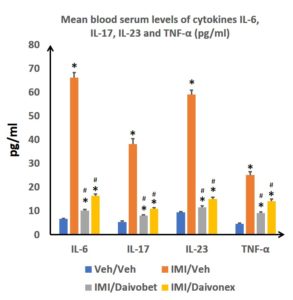
Figure 4: Graphic representation of cytokine level according to treatment administered – topical route: * p<0.05: test Post Hoc Student Newman Keuls vs. Veh/Veh; # p<0.05: test Post Hoc Student Newman Keuls vs. IMI/Veh)
c. Analysis of animal welfare and behaviour
We monitor animal well-being in an innovative way, by assessing the degree of interaction animals can achieve with their environment. The more an animal interacts with its environment, the more its well-being is enhanced. As shown in Figure 5, this evaluation allows us to identify the impact of active molecules on animal welfare.

Figure 5: Graphical representation of well-being score averages according to treatment administered and route of administration. (In IMI groups, the well-being score is significantly reduced in comparison to Veh/Veh groups. On Day 14, in all reference treatment groups, the well-being score is significantly improved in comparison with IMI/Veh groups)
In addition to assessing well-being, we carry out behavioural monitoring of locomotor activity, rearing and interactions with the affected skin area; for example, by counting the number of licks and scratches over a given period of time. Behavioural analysis helps strengthen the preclinical dossier for molecules in development, as illustrated on the Temisis poster.
3. Illustration of a study carried out by ETAP-Lab : TEM1657 is a new small molecule for the topical and oral treatment of psoriasis
This poster, presented by Temisis at the 54th International Conference on Medicinal Chemistry, in Strasbourg in 2018, uses in vivo data generated by the ETAP-Lab psoriasis model.
For more information, please see our dedicated page on dermatology models.
And if you’d like to know anything else about our work, please get in touch!







