Tau Clinical Trials – What can be learned from failure?
On the basis of the amyloid hypothesis of Alzheimer’s Disease (AD), most of the clinical trials conducted have concerned Aβ clearing therapy. Because of multiple failures in anti-amyloid clinical drug trials, more attention has been drawn to therapies targeted at tau in AD drug development. Disease-modifying therapies for AD and other tauopathies are targeting post-translational modifications, tau aggregation, microtubule stabilization, regulation of tau expression and tau clearance (immunotherapies). ClinicalTrials.gov currently lists about 120 studies targeting tau as a treatment for AD. Some drug candidates targeting tau are illustrated in the figure below, along with their current status in clinical trials.
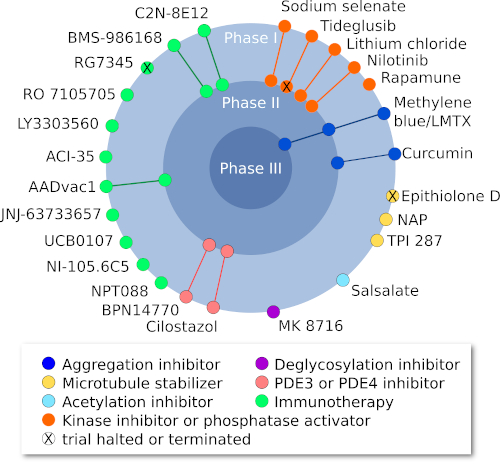
Figure 1: Current clinical trials targeting tau (Adapted from Congdon and Sirgudsson, 2018)
Contents
1. Targetable pathological events
1.1. Targeting post-translational modifications of tau
1.2. Targeting tau aggregation
1.3. Targeting microtubule stabilizers
1.4. Targeting tau expression
1.5. Immunotherapy (tau clearance)
1.6. Disease-modifying therapies using other mechanisms
2. What can be learned from clinical trial failure in tauopathies?
1.Targetable pathological events
1.1. Targeting post-translational modifications of tau
This aspect of tau-targeted therapies presented a major potential treatment strategy. It focused on post-translational tau modifications by:
- inhibiting tau hyper-phosphorylation kinases (e.g. GSK3β, CDK 5 and Fyn kinase)
- promoting the activity of tau dephosphorylation enzyme protein phosphate 2A (PP2A activators)
- inhibiting tau acetylation and tau deglycosylation
Unfortunately, no clinical benefits were observed. Since these processes begin long before symptoms of dementia become evident, clinical benefits might be observed if treatment could be administered earlier (Congdon & Sigurdsson, 2018).
1.2. Targeting tau aggregation
Despite promising preclinical results, both methylene blue and its derivative (TRx0237) have failed in clinical trials(Congdon & Sigurdsson, 2018). To gain insight into this failure, a recent study has shown that methylene blue actions are limited to inhibition of tau fibril formation, but do not have the same effect on tau oligomers (Soeda et al., 2019).
Curcumin, a natural polyphenolic molecule which can bind to proteins in β-sheet conformation and prevent aggregation, had no effect on either cognition or CSF biomarkers in two phase II trials in AD patients. Other clinical trials evaluating methylene blue’s derivative and curcumin are ongoing (Congdon & Sigurdsson, 2018).
1.3.Targeting microtubule stabilizers
Though microtubule-stabilizing drugs such as Epithilone D and abeotaxane (TPI 287) had shown preclinical promise, clinical trials of these stabilizers have been discontinued because of adverse effects (Varidaki et al., 2018). Davunetide (AL-108, NAP) is an eight amino acid peptide that has promoted microtubule stability and decreases tau phosphorylation in preclinical studies. MCI patients administered this peptide showed a trend towards improvements in working memory and attention in a phase II trial, but unfortunately, no significant differences relative to placebo were seen (Jarskog et al., 2013).
1.4. Targeting tau expression
Down-regulation of tau expression via antisense oligonucleotide technology has shown promising preclinical results. The phase II clinical trial on candidate therapeutics is ongoing in patients with Mild Alzheimer’s Disease (Jadhav et al., 2019).
1.5. Immunotherapy (tau clearance)
Emerging evidence in various preclinical models suggests that both active and passive immunotherapies are a practical approach to inducing antibody responses that are able to promote tau clearance. Many clinical trials evaluating immunotherapies are either ongoing, or set to enter, clinical trials in the near future (Congdon & Sigurdsson, 2018).
The anti-tau antibodies that have reached clinical trials target:
- Single or multiple phospho-epitopes
- Tau’s amino terminus
- Full-length normal and mutant tau
- Oligomeric tau
- Misfolded tau
Tau oligomers (TauO) are new therapeutic targets in AD. The targeting of TauO demands closer consideration than that of amyloid beta oligomers with regard to the variety of splicing isoforms, truncations, and post-translational modifications occurring naturally in the brain. Most clinical studies are currently in phase I or early phase II trials, and little data on efficacy is available (Vander Zanden & Chi, 2020).
1.6. Disease-modifying therapies using other mechanisms
A range of other approaches not specifically targeting tau are also under investigation (Huang et al., 2020). Some groups have turned their attention to protecting neurons from degeneration through the use of various methods, such as:
- Modulating components of the ubiquitin proteasome system or endoplasmic reticulum stress responses
- Neuroprotection
- Anti-inflammatory effects
- Growth factor promotion
- Metabolic effects (modifying physical activity, blood pressure and lipidemia)
- Stem cell therapies
Despite disappointing clinical trial outcomes, the therapeutic capacity of the drugs mentioned above is still far from being fully explored. To move the field forward, it is essential to learn from the failures of the past and use this knowledge to improve the clinical efficacy of these drugs (e.g. TRx0237, a derivative of methylene blue, will be studied at low doses. This trial is currently underway to confirm whether low-dose monotherapy is effective. Results are expected by the end of 2021).
2. What can be learned from clinical trial failure in tauopathies?
If it is true that we learn more from failure than from success, then we should accept, after a long series of failed trials for drugs targeting amyloid or tau deposits, that we lack understanding of certain critical aspects of disease biology that may affect the success of development programmes. The absence of an adequate understanding of the complex pathophysiology of AD seems to be mainly responsible for the failure of clinical trials (Kosik et al., 2016).
Removal of insoluble aggregates may be necessary, while not sufficient for therapeutic benefit. It is therefore reasonable to suggest that other, or additional, pathophysiological substrates need to be targeted (e.g. oligomeric forms that are considered the primary neurotoxic entity in AD, neuroinflammations and glial activation, autophagy/proteasome/unfolded protein response, oxidation, excitoxicity, iron deregulation, cholesterol metabolism, blood–brain barrier dysfunction, etc.) (Lo et al., 2014; Kosik et al., 2016; Galzitskaya, 2020).
Most tau-targeting therapies in clinical trials are immunotherapies (Congdon & Sigurdsson, 2018). Many factors govern the efficacy of anti-tau therapeutic antibodies, such as the epitope (normal or primarily pathological) and the site of action (extra- and intra-cellular, or extracellular only).
The lack of success of AD studies has also raised the question of whether the stage of disease generally targeted (mild-to-moderate dementia stages) may be too late. Aggregate deposition begins 10 years or more prior to the onset of cognitive symptoms. Earlier diagnosis and treatment of the disease might be more effective (Lasagna-Reeves et al., 2012; Combs et al., 2016).
Biomarkers play a critical role in the success of drug development programmes. The use of various types of biomarkers is associated with higher success rates in trials, in comparison with trials having no or few biomarkers (Green et al., 2018). AD clinical trials have commonly used cognitive and function improvement as co-primary endpoints (Sabbagh et al., 2019). In addition to these biomarkers, others must be further investigated in the trial process, such as biomarkers for neuroinflammation and glial activation, axonal degeneration, synaptic dysfunction, iron metabolism and excessive oxidation. The combination of these biomarkers allows more precise staging of AD (Anoop et al., 2010; Blennow et al., 2010).
New clinical trial designs (including adequate trial and appropriate population sizes, dosage of study drug and biomarker selection) will need to be further optimised to detect the effects of disease-modifying drugs (Romero et al., 2015; Das & Lo, 2017). Furthermore, tools used in AD studies having enough sensitivity to detect differences between study drug and placebo must be further developed.
Animal models are important gateways in the drug development process. The use of transgenic mice as a model system in preclinical testing may be a contributing factor to the high failure rate of AD trials. The reproducibility and translatability of preclinical models are urgently needed to improve both predictability and efficiency along the critical path (Seyhan, 2019). Better translational models of AD, which accurately portray the complexity of sporadic forms, can contribute to greater success in AD drug development (approaches include the use of human induced pluripotent stem cells, wild-type animals, etc.).
It remains possible that targeting a single pathologic pathway is inadequate, due to both the complex pathophysiology of AD and to the fact that combination therapy targeting multiple pathological proteins (such as amyloid beta or tau) or multiple compounds having different mechanisms of action, is necessary to success (Art 4). It remains possible that, given the great complexity of Alzheimer’s disease, targeting a single pathological pathway is inadequate. The use of therapies acting on several pathological pathways, via the combination of treatments (targeting, for example, amyloid beta and tau) may be necessary to success (see Article 4 for more information).
Complementary articles about Tau and Tau oligomers:
6 reasons to include tau oligomers in your R&D strategy for the treatment of Alzheimer’s Disease
New hope for Alzheimer’s disease: what’s combining therapeutic strategies all about?
More information about our neurodegenerative diseases models:
References
Anoop, A., Singh, P.K., Jacob, R.S., & Maji, S.K. (2010) CSF Biomarkers for Alzheimer’s Disease Diagnosis. Int J Alzheimers Dis, 2010.
Blennow, K., Hampel, H., Weiner, M., & Zetterberg, H. (2010) Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol, 6, 131–144.
Combs, B., Hamel, C., & Kanaan, N.M. (2016) Pathological conformations involving the amino terminus of tau occur early in Alzheimer’s disease and are differentially detected by monoclonal antibodies. Neurobiol Dis, 94, 18–31.
Congdon, E.E. & Sigurdsson, E.M. (2018) Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol, 14, 399–415.
Das, S. & Lo, A.W. (2017) Re-inventing drug development: A case study of the I-SPY 2 breast cancer clinical trials program. Contemp Clin Trials, 62, 168–174.
Galzitskaya, O.V. (2020) Oligomers Are Promising Targets for Drug Development in the Treatment of Proteinopathies. Front Mol Neurosci, 12.
Green, D.J., Liu, X.I., Hua, T., Burnham, J.M., Schuck, R., Pacanowski, M., Yao, L., McCune, S.K., Burckart, G.J., & Zineh, I. (2018) Enrichment Strategies in Pediatric Drug Development: An Analysis of Trials Submitted to the US Food and Drug Administration. Clin Pharmacol Ther, 104, 983–988.
Huang, L.-K., Chao, S.-P., & Hu, C.-J. (2020) Clinical trials of new drugs for Alzheimer disease. J Biomed Sci, 27, 18.
Jadhav, S., Avila, J., Schöll, M., Kovacs, G.G., Kövari, E., Skrabana, R., Evans, L.D., Kontsekova, E., Malawska, B., de Silva, R., Buee, L., & Zilka, N. (2019) A walk through tau therapeutic strategies. Acta Neuropathologica Communications, 7, 22.
Jarskog, L.F., Dong, Z., Kangarlu, A., Colibazzi, T., Girgis, R.R., Kegeles, L.S., Barch, D.M., Buchanan, R.W., Csernansky, J.G., Goff, D.C., Harms, M.P., Javitt, D.C., Keefe, R.S., McEvoy, J.P., McMahon, R.P., Marder, S.R., Peterson, B.S., & Lieberman, J.A. (2013) Effects of Davunetide on N -acetylaspartate and Choline in Dorsolateral Prefrontal Cortex in Patients with Schizophrenia. Neuropsychopharmacology, 38, 1245–1252.
Kosik, K.S., Sejnowski, T.J., Raichle, M.E., Ciechanover, A., & Baltimore, D. (2016) A path toward understanding neurodegeneration. Science, 353, 872–873.
Lasagna-Reeves, C.A., Castillo-Carranza, D.L., Sengupta, U., Sarmiento, J., Troncoso, J., Jackson, G.R., & Kayed, R. (2012) Identification of oligomers at early stages of tau aggregation in Alzheimer’s disease. FASEB J, 26, 1946–1959.
Lo, A.W., Ho, C., Cummings, J., & Kosik, K.S. (2014) Parallel Discovery of Alzheimer’s Therapeutics. Science Translational Medicine, 6, 241cm5-241cm5.
Romero, K., Ito, K., Rogers, J.A., Polhamus, D., Qiu, R., Stephenson, D., Mohs, R., Lalonde, R., Sinha, V., Wang, Y., Brown, D., Isaac, M., Vamvakas, S., Hemmings, R., Pani, L., Bain, L.J., Corrigan, B., Alzheimer’s Disease Neuroimaging Initiative, & Coalition Against Major Diseases (2015) The future is now: model-based clinical trial design for Alzheimer’s disease. Clin Pharmacol Ther, 97, 210–214.
Sabbagh, M.N., Hendrix, S., & Harrison, J.E. (2019) FDA position statement “Early Alzheimer’s disease: Developing drugs for treatment, Guidance for Industry.” Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 5, 13–19.
Seyhan, A.A. (2019) Lost in translation: the valley of death across preclinical and clinical divide – identification of problems and overcoming obstacles. Translational Medicine Communications, 4, 18.
Soeda, Y., Saito, M., Maeda, S., Ishida, K., Nakamura, A., Kojima, S., & Takashima, A. (2019) Methylene Blue Inhibits Formation of Tau Fibrils but not of Granular Tau Oligomers: A Plausible Key to Understanding Failure of a Clinical Trial for Alzheimer’s Disease. J Alzheimers Dis, 68, 1677–1686.
Vander Zanden, C.M. & Chi, E.Y. (2020) Passive Immunotherapies Targeting Amyloid Beta and Tau Oligomers in Alzheimer’s Disease. Journal of Pharmaceutical Sciences, 109, 68–73.
Varidaki, A., Hong, Y., & Coffey, E.T. (2018) Repositioning Microtubule Stabilizing Drugs for Brain Disorders. Front Cell Neurosci, 12.